Greenhouse gases are a group of compounds that are able to trap heat (longwave radiation) in the atmosphere, keeping the Earth's surface warmer than it would be if they were not present. These gases are the fundamental cause of the greenhouse effect. Increases in the amount of greenhouse gases in the atmosphere enhances the greenhouse effect which is creating global warming and consequently climate change.
Greenhouse gases allow sunlight (shortwave radiation) to pass through the atmosphere freely, where it is then partially absorbed by the surface of the Earth. But some of this energy bounces back out towards space as heat. Of the heat emitted back to space, some is intercepted and absorbed by greenhouse gases in the atmosphere. This is because these compounds are made of three or more atoms. This molecular structure allows them to absorb some of the escaping heat and then re-emit it towards the Earth which increases global temperatures.
The ability of these gases to trap heat is what causes the greenhouse effect. So the more greenhouse gases you have in the atmosphere, the more heat stays on Earth. This process, which is very similar to the way a greenhouse works, is why the gases that can produce this effect are collectively known as greenhouse gases.
There are 2 ways that a greenhouse gas (often abbreviated GHG) can enter our atmosphere. One of them is through human activities. The main human sources of GHG emissions are: fossil fuel use, deforestation, intensive livestock farming, use of synthetic fertilizers and industrial processes. The other is through natural processes like animal and plant respiration.
The principal forcing greenhouse gases are:
The main feedback greenhouse gas is:
Figure 1: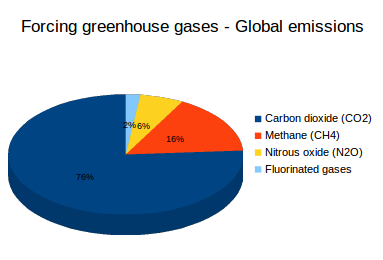
Source: Forcing greenhouse gases - Global emissions (2012), International Energy Agency.
Forcing greenhouse gases take many years to leave the atmosphere. CO2, CH4, N2O and the fluorinated gases are all well-mixed gases in the atmosphere. They do not react to changes in either temperature or air pressure and thus do not get removed easily like water that condenses to become rain or snow. Their long atmospheric lifetimes allows them to have a lasting effect on global warming and climate change.
Water vapor on the other hand has a residence time of a few days. It is a highly active component of the climate system that responds rapidly to changes in conditions by either condensing into rain or snow, or evaporating to return to the atmosphere. Thus the impact of the greenhouse effect is primarily circulated through water vapor, and it acts as a fast feedback, accentuating the warming provided by the forcing greenhouse gases.
CO2 and the other forcing greenhouse gases are the key gases within the Earth's atmosphere that sustain the greenhouse effect and control its strength. In fact, the greenhouse effect would collapse were it not for the presence of CO2 and the other non-condensing greenhouse gases.
Greenhouse gases greatly affect the temperature of the Earth. Without them, surface temperatures would be on average about 32.5°C colder than the present average of 14.4°C (57.9°F), making life on Earth as we know it not possible. Greenhouse gases are not, inherently, a bad thing. But the growing concentration of greenhouse gases in the atmosphere has been raising average temperatures around the world.
CO2, CH4 and N2O are emitted to the atmosphere through natural processes as well as human activities (use of fossil fuels, industrial production, etc). The fluorinated gases on the other hand, are created and emitted almost exclusively through human activities.
Since the Industrial Revolution, which began in the 18th century, human activities have been a major source of all forcing greenhouse gases. Human activities have led to a sharp and dangerous increase of these gases within the Earth's atmosphere, so much so that the growth of all forcing greenhouse gas (GHG) concentrations is now directly controlled by humans.

