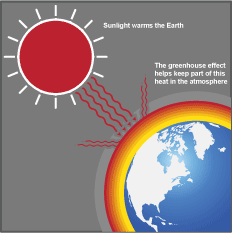
The greenhouse effect increases the temperature of the Earth by trapping heat in our atmosphere. This keeps the temperature of the Earth higher than it would be if direct heating by the Sun was the only source of warming. When sunlight reaches the surface of the Earth, some of it is absorbed which warms the ground and some bounces back to space as heat. Greenhouse gases that are in the atmosphere absorb and then redirect some of this heat back towards the Earth.
The greenhouse effect is a major factor in keeping the Earth warm because it keeps some of the planet's heat that would otherwise escape from the atmosphere out to space. In fact, without the greenhouse effect the Earth's average global temperature would be much colder and life on Earth as we know it would not be possible. The difference between the Earth's actual average temperature 14° C (57.2° F) and the expected effective temperature just with the Sun's radiation -19° C (-2.2° F) gives us the strength of the greenhouse effect, which is 33° C.
The greenhouse effect is a natural process that is millions of years old. It plays a critical role in regulating the overall temperature of the Earth. The greenhouse effect was first discovered by Joseph Fourier in 1827, experimentally verified by John Tyndall in 1861, and quantified by Svante Arrhenius in 1896.
How does the greenhouse effect work?
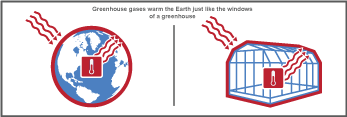
To understand exactly how the greenhouse effect works, imagine the following: a warm, sunny day where the sun shines bright on the Earth. This sunlight (shortwave radiation) passes into the planet's atmosphere and warms the Earth. Part of this energy is absorbed by the Earth's surface, transformed into heat (longwave radiation) and radiated back towards space. But as this heat goes up through the atmosphere, some of it is trapped by the different greenhouse gases and doesn't escape into space. This in turn warms up the Earth's atmosphere; just like the windows of a greenhouse that lets light in and keeps the heat within to warm the plants growing inside.
Since some of the heat can't escape into space, it continues to add up which then warms up the Earth. This is what we call the greenhouse effect. So the more greenhouse gases you have in the atmosphere, the more heat stays on Earth.
If the amount of energy from the sun and the amount of greenhouse gases in the atmosphere remain the same, then the average temperature on Earth will also be constant. But this is no longer the case. The amount of greenhouse gases in our atmosphere is the highest it has been in the last 3 million years. This is enhancing the greenhouse effect and making the Earth warmer than normal, which is affecting the planet's weather patterns, creating global warming and climate change.
An everyday example of the greenhouse effect
If you open the door of a car that has been left parked in the sun for a couple of hours, you'll notice that the temperature inside the car is much warmer than the temperature outside. This is because the windows of the car allow the sunlight to enter. This light, once inside, is then partially converted into heat. However, these same windows do not allow the heat inside the car to pass through as easily as light, so some of this heat accumulates. The net effect is that more heat remains in than can come out, increasing the temperature inside the car.
The greenhouse effect is caused by the interaction of the sun's energy with greenhouse gases such as carbon dioxide, methane, nitrous oxide and fluorinated gases in the Earth's atmosphere. The ability of these gases to trap heat is what causes the greenhouse effect.
Greenhouse gases are made of three or more atoms. This molecular structure makes it possible for these gases to trap heat in the atmosphere and then re-emit it towards the surface which further warms the Earth. This continuous cycle of trapping heat leads to an overall increase in global temperatures. This process, which is very similar to the way a greenhouse works, is why the gases that can produce this effect are collectively known as greenhouse gases.
The principal forcing gases of the greenhouse effect are:
The main feedback gas of the greenhouse effect is:
Carbon dioxide, methane, nitrous oxide and the fluorinated gases are all well-mixed gases in the atmosphere that do not react to changes in temperature and air pressure, so the levels of these gases are not affected by condensation. Water vapor on the other hand, is a highly active component of the climate system that responds rapidly to changes in conditions by either condensing into rain or snow, or evaporating to return to the atmosphere. Thus the impact of the greenhouse effect is primarily circulated through water vapor, and it acts as a fast feedback.
Carbon dioxide and the other non-condensing greenhouse gases are the key gases within the Earth's atmosphere that sustain the greenhouse effect and control its strength. Water vapor is a fast-acting feedback but its atmospheric concentration is controlled by the radiative forcing supplied by the non-condensing greenhouse gases.
In fact, the greenhouse effect would collapse were it not for the presence of carbon dioxide and the other non-condensing greenhouse gases. Together the feedback by the condensing and the forcing by the non-condensing gases within the atmosphere both play an important role in the greenhouse effect.