There are 2 ways that greenhouse gas emissions enters our atmosphere. One of them is through human activities. The main human sources of greenhouse gas emissions are: fossil fuel use, deforestation, intensive livestock farming, use of synthetic fertilizers and industrial processes. The other is through natural processes like animal and plant respiration.
There are four main types of forcing greenhouse gases: carbon dioxide, methane, nitrous oxide and fluorinated gases. The main feedback greenhouse gas is water vapor.
Greenhouse gas emissions trap heat in the Earth's atmosphere, just as the glass of a greenhouse keeps warm air inside. Human activity increases the amount of greenhouse gas emissions entering in the atmosphere, contributing to a warming of the Earth's surface.
Let's take a closer look at the sources of greenhouse gas emissions:
There are both natural and human sources of carbon dioxide (CO2) emissions. Natural sources include decomposition, ocean release, respiration and volcanoes. Human sources come from activities like cement production, deforestation and the burning of fossil fuels.
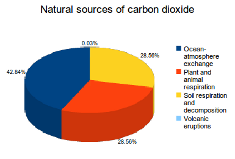
42.8 percent of all naturally produced CO2 emissions come from ocean-atmosphere exchange. Other important natural CO2 sources include plant and animal respiration (28.56%) as well as soil respiration and decomposition (28.56%). A minor amount is also created by volcanic eruptions (0.03%).
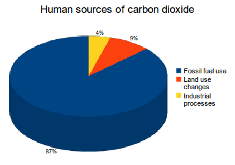
87 percent of all human CO2 emissions come from the burning of fossil fuels like coal, natural gas and oil. Other sources include deforestation (9%), and industrial processes such as cement manufacturing (4%).
Human sources of CO2 are much smaller than natural emissions but they upset the balance in the carbon cycle that existed before the Industrial Revolution. The amount of CO2 produced by natural sources is completely offset by natural carbon sinks and has been for thousands of years.
Before the influence of humans, CO2 levels were quite steady because of this natural balance. Since the Industrial Revolution, human sources of CO2 emissions have been growing. Activities such as the burning of fossil fuels as well as deforestation are the primary cause of the increased CO2 concentrations in the atmosphere.
While there are both natural and human sources of methane (CH4), humans create the majority of total emissions. The main natural sources include wetlands, termites and the oceans. Important human sources come from landfills, livestock farming, as well as the production, transportation and use of fossil fuels.
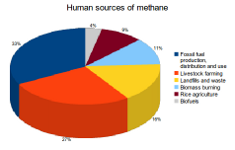
Human-caused emissions have increased greatly since the Industrial Revolution. Activities such as fossil fuel production and intensive livestock farming are the primary cause of the increased CH4 concentrations in the atmosphere. Together these two sources are responsible for 60% of all human CH4 emissions. Other sources include landfills and waste (16%), biomass burning (11%), rice agriculture (9%) as well as biofuels (4%).
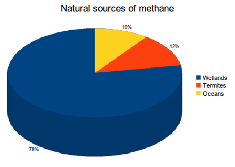
78% of natural CH4 emissions are produced by wetlands. Other natural CH4 sources include termites (12%) and the oceans (10%).
For thousands of years, natural CH4 sources have been closely balanced by natural sinks. But today, human-related sources create the majority of total CH4 emissions. This has upset the natural balance that existed before the Industrial Revolution and is increasing atmospheric levels.
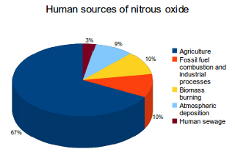
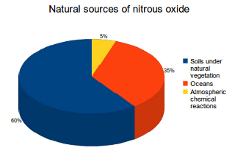
Nitrous oxide (N2O) emissions are also produced by both natural and human sources. The main natural sources are soils under natural vegetation and the oceans. Important human sources come from agriculture, fossil fuel combustion and industrial processes.
Human activities such as agriculture, fossil fuel use and industrial processes are the primary cause of the increased N2O concentrations in the atmosphere. Together these sources are responsible for 77% of all human N2O emissions. Other sources include biomass burning (10%), atmospheric deposition (9%) and human sewage (3%).
60% of natural N2O emissions are produced by soils under natural vegetation. Other natural sources include the oceans (35%) and atmospheric chemical reactions (5%).
Human N2O sources are smaller than natural emissions. But increasing emissions from human sources have upset the balance in the nitrogen cycle that existed before the Industrial Revolution. For thousands of years, natural N2O sources have been closely balanced by natural sinks. Before the influence of humans, N2O levels were quite steady because of this natural balance.
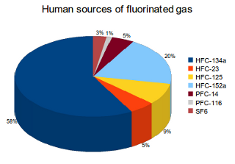
Emissions of the three main fluorinated gases (hydrofluorocarbons (HFCs), perfluorocarbons (PFCs) and sulphur hexafluoride (SF6)) are almost all created by humans and are used mainly in industrial processes. With the exception of PFC-14 (CF4), fluorinated gases have no natural sources.
HFCs are the largest source of fluorinated gas emissions, accounting for 91%. HFCs are used inside of products like refrigerators, air-conditioners, foams and aerosol cans. Emissions from these products are caused by gas leakage during the manufacturing process as well as throughout the product's life. If disposal is not done properly, HFCs continue to leak out of the product until they are empty.
PFCs are responsible for 6% of fluorinated gas emissions. These gases are created during the production processes of aluminum and semiconductors. PFC-14 (CF4) and PFC-116 (C2F6) account for the majority of PFC emissions. Less than 0.1% of PFC emissions is caused by natural sources. Small amounts of CF4 are found in fluorite, granite and natural gas deposits. Geochemical reactions in the lithosphere cause these emissions.
SF6 creates 3% of fluorinated gas emissions. This gas is mainly used by the electric power industry as an insulator and arc interrupter. The other important source of SF6 emissions is from its use as a cover gas in magnesium production.
The increase in the atmospheric levels of fluorinated gases has been caused exclusively by human emissions. For a long time now human sources of fluorinated gases have been creating emissions much more rapidly than the Earth can remove them.
Water Vapor
The atmospheric concentration of water vapor is highly variable and depends largely on temperature. Water vapor is a highly active component of the climate system that responds rapidly to changes in conditions by either condensing into rain or snow, or evaporating to return to the atmosphere. The water content of the atmosphere is constantly being depleted by precipitation as well as being replenished by its main source, evaporation from seas, lakes, rivers, and moist earth.
Human activity does not significantly affect water vapor concentrations except at local scales, such as near irrigated fields. Since its concentration is controlled by the climate itself, water vapor acts as a fast feedback, reacting to, and amplifying the warming provided by the forcing greenhouse gases.
More info:
Greenhouse Gas Emissions: Causes & Sources - Live Science
Sources of Greenhouse Gas Emissions - US EPA
Greenhouse Gas Inventory Data - United Nations Framework Convention on Climate Change
Greenhouse gas emissions by industries and households - Eurostat